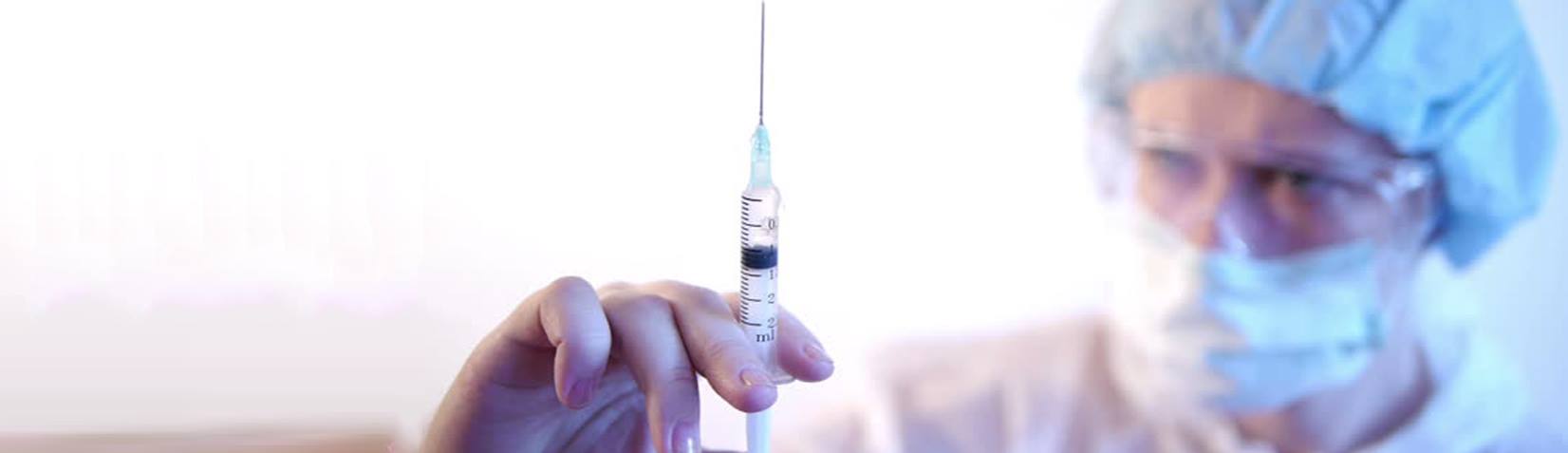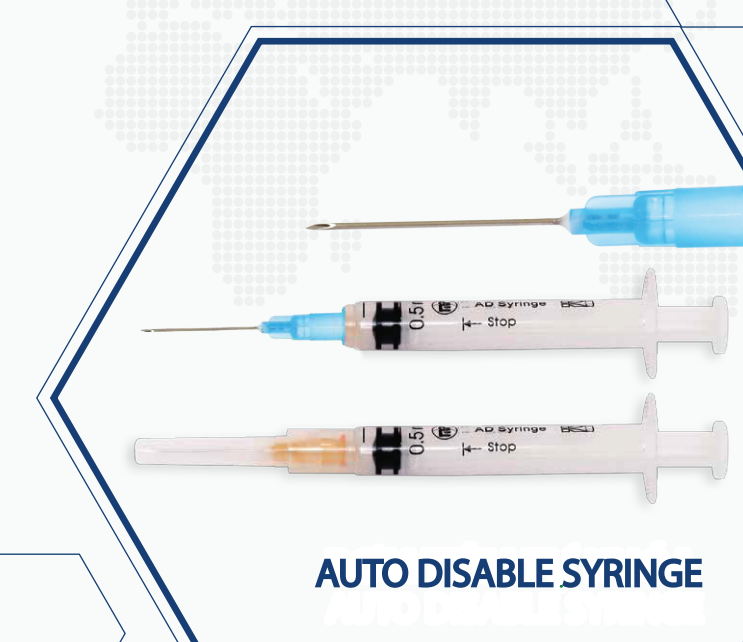
Auto-disable 0.5ml syringe
Auto-disable syringes are manufactured on Japanese equipment and technology in accordance with international copyright and patents issued by Star, sterilized by E.O gas, sterile, non-toxic and non-infectious.
Auto-disable syringes have been certified by WHO in accordance with E8/DS1 standard since 2005, PQS E8/26 (http://www.who.int)
Technical specification:
Syringe reached the required on the physical, chemical and biological ISO 7886-3: 2005
Syringe needle inserted pliers: made of stainless steel, suitable properties of a material according to ISO 7864:1993 (E) and ISO 9626:1991
Sterilized by E.O gas: achieving ISO 11138-1: 2006 (E) and ISO 11138-2:2006 (E)
Quality management system: ISO 9001:2008 and ISO 13485:2012
Shelf life: 3 years
Packaging: accordance with the requirements of ISO 11607:1997
Packing type: 100 syringesin a box of Chipbox. 24 Chipbox boxes in a carton
Products labels: information on the appropriate product standards BSEN 1041:1998 and NS-EN 980:2003
| DOWNLOAD BROCHURE |
| Contact for OEM: sale.world@vinamed.com.vn |